How to Mix Salt Nacl Sand Aluminum Iron and Then Separate Again
In this class experiment, students divide a mixture of sand and salt, illustrating the key means of separating a mixture of an insoluble material from 1 that is soluble
This is a very straightforward experiment. It can be carried out individually or in groups of ii. Pupils must stand up during heating activities and beware of hot salt spitting when evaporation is near complete.
Equipment
Apparatus
- Eye protection
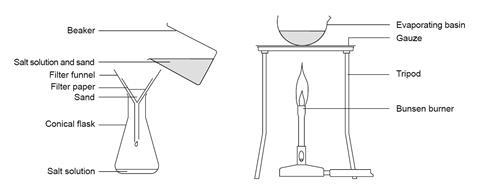
- Beaker, 250 cm3
- Glass stirring rod
- Filter funnel
- Filter paper
- Conical flask, 250 cm3
- Evaporating basin
- Bunsen burner
- Heat resistant mat
- Tripod
- Gauze
Chemicals
- Mixture of sand and sodium chloride (common salt), well-nigh 6–seven yard per group of students (a suitable sand–salt mixture should contain approximately xx% table salt by mass)
Health, safety and technical notes
- Wearable eye protection throughout this experiment.
- Pupils must stand up during heating activities and beware of hot salt spitting when evaporation is almost complete.
- Sodium chloride (eg table salt), NaCl(due south) - encounter CLEAPSS Hazcard HC047b.
Process
- Cascade the sand–salt mixture into the chalice so that information technology just covers the base of operations.
- Add well-nigh 50 cm3 of water, or add water until the beaker is near i-5th full.
- Stir the mixture gently for a few minutes.
- Filter the mixture into a conical flask.
- Cascade the filtrate into an evaporating basin.
- Heat the table salt solution gently until information technology starts to decrepitate (spit). CARE: Keep eye protection on and do not get too close.
- Turn off the Bunsen burner and let the clammy common salt dry in the dish.
Teaching notes
If desired, the experiment tin can be extended to isolate dry samples of sand and salt. To do this, the damp sand in the filter paper can exist transferred to another canvass of dry filter paper, and, by folding and dabbing, the sample can be dried. If necessary, another piece of filter newspaper can be used.
Students frequently like to nowadays their specimens in small bottles for blessing, so a spatula could be used to accomplish this. While the starting time student of a pair is transferring the sand, the other can be scraping the dried common salt from the evaporating dish and transferring it to another specimen bottle.
If this extension is carried out, the students should be encouraged to characterization the bottles. They should be told that all samples prepared in this mode demand to be labelled, fifty-fifty if in this case, it should be obvious which substance is which.
Student questions
- Why can sand and salt be separated using this experiment?
- Why is the salt, sand and water mixture stirred in stride 3?
- Why is the salt solution heated in step half-dozen?
- How might the final traces of water exist removed from your samples to ensure that they are totally dry?
- Give ii reasons why the sand you have obtained might yet be contaminated with salt.
- How could you adapt your experiment to obtain a purer sample of sand?
- Give two reasons why the salt you have obtained might still exist contaminated with sand.
- How could you lot adapt your experiment to obtain a purer sample of salt?
Primary science teaching notes
If you teach chief science, the following information is designed to help you use this resource.
Skill development
Children will develop their working scientifically skills past:
- Drawing conclusions and raising further questions that could be investigated, based on their information and observations.
- Using appropriate scientific language and ideas to explicate, evaluate and communicate their methods and findings.
Learning outcomes
Children will:
- Observe that some materials volition dissolve in liquid to form a solution.
- Describe how to recover a substance from a solution.
- Apply cognition of solids, liquids and gases to decide how mixtures might be separated, including through filtering, sieving and evaporating.
- Demonstrate that dissolving, mixing and changes of state are reversible changes.
Concepts supported
Children volition acquire:
- That in that location are diverse techniques that can exist used to separate different mixtures.
- That dissolving is a reversible reaction.
- That not all solids are soluble.
- That the rate of dissolving can be affected by various factors.
- That melting and dissolving are not the same process.
Suggested activity use
This activity can be used as a whole-class investigation, with children working in small groups or pairs to look at how to dissever the table salt and sand. This could provide a stimulus for further investigations looking at how to separate other mixtures of solids, either of unlike particle sizes or by solubility.
Practical considerations
Primary schools frequently don't have Bunsen burners, and then viable alternatives demand to be sourced. Similarly, it may be difficult to source the equipment needed to evaporate water to recover the dissolved salt. Head stands and tea lights tin piece of work well every bit possible alternatives.
When carrying out this activity exist aware that some insoluble solids are able to form suspensions. This is where the particles appear to accept dissolved, when in fact they have been spread out throughout the liquid. A good indicator that a suspension has formed is that the liquid will become cloudy or the particles tin be heard scraping as the mixture is stirred.
The layout of this activity is very prescriptive as the procedure is set up out on a footstep by step ground. An open up challenge activity, with children working in small groups and devising their own methods, would extend the children's thinking. Different groups' suggestions could be compared and evaluated as a grade.
Source: https://edu.rsc.org/experiments/separating-sand-and-salt-by-filtering-and-evaporation/386.article
0 Response to "How to Mix Salt Nacl Sand Aluminum Iron and Then Separate Again"
Post a Comment